44 regulations require that product labels on containers
Federal Hazardous Substances Act (FHSA) Requirements Whether a product must be labeled depends on its contents and the likelihood that consumers will be exposed to any hazards it presents. To require labeling, a product must first be toxic, corrosive, flammable or combustible, an irritant, or a strong sensitizer, or it must generate pressure through decomposition, heat, or other means. eCFR :: 9 CFR Part 317 -- Labeling, Marking Devices, and Containers Labeling, filling of containers, handling of labeled products to be only in compliance with regulations. § 317.12: Relabeling products; requirements. § 317.13: Storage and distribution of labels and containers bearing official marks. §§ 317.14-317.15 [Reserved] § 317.16: Labeling and containers of custom prepared products. § 317.17
eCFR :: 9 CFR Part 112 -- Packaging and Labeling ( i) Only the VLN or VPN is required on container labels of interchangeable (non-critical) components of diagnostic kits and container labels for individual products packaged together for co-administration. ( ii) The PCN may be used in lieu of the true name of the kit on small container labels for critical components of diagnostic kits.

Regulations require that product labels on containers
Quality System Regulation Labeling Requirements | FDA Various sections of the QS regulation have an impact on labeling: Section 21 CFR 820.80 (b) requires the inspection and testing of incoming materials including labeling; and 21 CFR 820.70 (f ... 1998 ap statistics Regulations require that product labels on containers of food that are available ... Specifically, if milk containers are labeled to have 128 fluid ounces. Compliance FAQs: Packaging and Labeling in the US | NIST title 19, united states code, chapter 4, section 1304 and 19 cfr 134, country of origin marking regulations require that every article of foreign origin (or its container) imported into the u.s. be marked in a conspicuous place as legibly, indelibly, and permanently as the nature of the article (or container) will permit, and in such a manner as …
Regulations require that product labels on containers. Name! That! Test! Flashcards - Quizlet Regulations require that product labels on containers of food that are available for sale accurately state the amount of food in those containers. if milk ... Chemical Container Labels | EHS - University of Washington Laboratories can print their own labels or obtain labels from EH&S by calling 206.616.0585. Labels for UW-synthesized chemicals. University researchers who synthesize a new chemical or product need to generate labels for containers holding the chemical/product that includes chemical information and any known or suspected hazards. CPSC Labeling Requirements Overview Federal Hazardous Substances Act (FHSA). The FHSA requires precautionary labeling on the immediate container of hazardous household substances to help consumers ... Packaging Materials Regulations in the United States: An Overview The FPLA sets a series of standards for product labeling contents in terms of wording, typesetting, design, and units. Here are some of the requirements: 1. Identity of the commodity 2. Name and place of business of the manufacturer, packer, or distributor 3.
FDA Food Packaging Guidelines for 2022 | Newprint Canadian food labeling regulations also require the name of the product and net quantity to be printed on the PDP. However, other required information can be placed anywhere on the packaging and they include: Common name Net quantity Information about primary business or dealer Storage instructions (If food is not to be stored at room temperature) PDF Industry Guide To Marketing Container Plants The required information may appear on either signage or container labels. Retailers can decide which method (sign or label) is preferred and that method may vary among separate plant displays in the same store. The declaration of identity and net contents must appear together, on either signage or labeling as described below. Chapter 6: Product Labeling, Regulations, & Label Design & Packaging The regulations focus on eight major food allergens: milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, and soybean. Sesame is being added in 2023. Until that time, you don't have to list sesame as an allergen, although it will appear in your ingredient statement unless it's part of a seasoning. You can find FDA guidance here. Marijuana Packaging & Labeling Laws by State | Leafly General Label Requirements; Prohibitions; Exceptions(1) Principal Display Panel.(a) Every container that contains a marijuana item for sale or transfer to a consumer, patient or designated primary ...
Hazmat Label Requirements and Regulations - Where Regulations Require ... The label must be printed on or affixed to a surface of the package or containment. The label must be located on the same surface of the package and near the shipping name marking. If primary and subsidiary hazard labels are required, they must be displayed with 6 inches of each other. Duplicate Labeling Necessary for: US Product Labeling Laws and Regulations - The Bayne Law Group, LLC The Federal Hazardous Substances Act (FHSA) requires precautionary labeling on the immediate container of hazardous household products to help consumers safely store and use those products and to give them information about immediate first aid steps to take if an accident happens. Product Labeling Laws - Explained - The Business Professor, LLC The FHSA is a federal law administered by the CPSC. The FHSA requires labeling of containers of hazardous products. The label must provide notice of the potentially harmful effects of contact with the hazardous substance and the first aid steps to take in the event of exposure. Pursuant to this Act, the CPSA may ban products that are ... California Code of Regulations, Title 8, Section 5194. Hazard ... Sep 28, 2018 · (B) Any food, food additive, color additive, drug, cosmetic, or medical or veterinary device, including materials intended for use as ingredients in such products (e.g., flavors and fragrances), as such terms are defined in the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 301 et seq.) and regulations issued under that Act, when they are subject to the labeling requirements of that Act and ...
EU - Labeling/Marking Requirements Aug 11, 2022 ... The product function and list of ingredients must appear also on the container or packaging. Member States may stipulate that the information on ...
Packaging, Labeling, Transporting, Storing — Food Law 21 CFR 130.14 (b) regulates the labeling of food product of "substandard quality" and "substandard fill." . A second consideration with respect to packaging is whether the container may cause the food to be adulterated. Packaging materials are considered an "indirect food additive;" see 21 CFR parts 174-178 .
Shelf Life and Expiration Dating of Cosmetics | FDA There are no U.S. laws or regulations that require cosmetics to have specific shelf lives or have expiration dates on their labels. However, manufacturers are responsible for making sure their ...
Bag Regulations and Labeling Rules in the United States: An Overview A COO (Country of Origin label) is obligatory to be attached on most of the products in the US market, including handbags, backpacks and other related products. The product and the packaging must have a permanently affixed country of origin label. A product packing is not appropriate and qualified with merely stickers on. Examples Made in China
Labeling outer containers of chemical cleaning products while in ... Response: Paragraph 29 CFR 1910.1200 (f) (1) requires manufacturers (and importers and distributors) to ensure that each shipped container leaving the workplace is labeled, tagged, or marked in accordance with paragraphs (f) (1) (i)- (vi).
Solved Regulations require that product labels on containers | Chegg.com Specifically, if milk containers are labeled to have 128 fluid ounces and the mean number of fluid ounces of milk in the containers is at least 128, the milk processor is considered to be in compliance with the regulations. The filling machines can be set to the labeled amount.
Frequently Asked Questions on the Container and Containment Labeling ... Generally, no. The language required by the container and containment regulations adds to the statements currently on the pesticide label. However, in many cases, it would make sense to work the required language into the existing container disposal section. For example, "Triple rinse (or equivalent)." may appear on many product labels.
Labeling Requirements | US EPA The label on a pesticide package or container and the accompanying instructions are a key part of pesticide regulation. The label provides critical information about how to handle and safely use the pesticide product and avoid harm to human health and the environment. Labeling Requirement Resources
Labelling and packaging - Trade - European Commission In the case of pre-packaged products, operators are required to state on a label ... the presentation and labelling requirements set out in Regulation (EU) ...
Labeling Regulations for Chemical Transportation - OnlineLabels Labeling Regulations for Chemical Transportation. During the supply chain, your products pass through a variety of different hands. With the transportation of dangerous goods in particular, manufacturers are required to follow specific labeling regulations. These requirements are designed to keep the employees, carriers, and transporters ...
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (d) Labels and other labeling materials for each different drug product, strength, dosage form, or quantity of contents shall be stored separately with suitable identification. Access to the storage area shall be limited to authorized personnel. (e) Obsolete and outdated labels, labeling, and other packaging materials shall be destroyed.
Cosmetics Labeling Guide | FDA Regulations require that "[the label of a cosmetic product shall bear a warning statement whenever necessary or appropriate to prevent a health hazard that may be associated with the product" [21 ...
Household Cleaners: How to Properly Label a Cleaning Product Container Cleaning and disinfectant product labels have to be eye-catching to potential customers, withstand constant handling, adhere to containers for months or even years, and endure contact with the cleaner or disinfectant itself. If that weren't enough, the ink must resist fading and smearing to ensure safety information is always legible.
Labeling of Secondary Containers | Occupational Safety and Health ... section 1910.1200 (f) (6) (ii) requires that workplace labeling include "product identifier and words, pictures, symbols, or combination thereof, which provide at least general information regarding the hazards of the chemicals, and which, in conjunction with the other information immediately available to employees under the hazard communication …
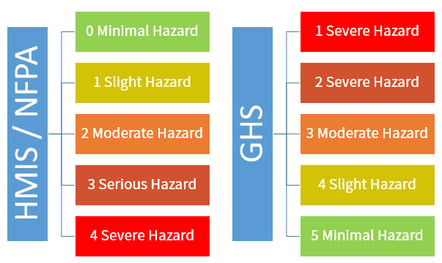
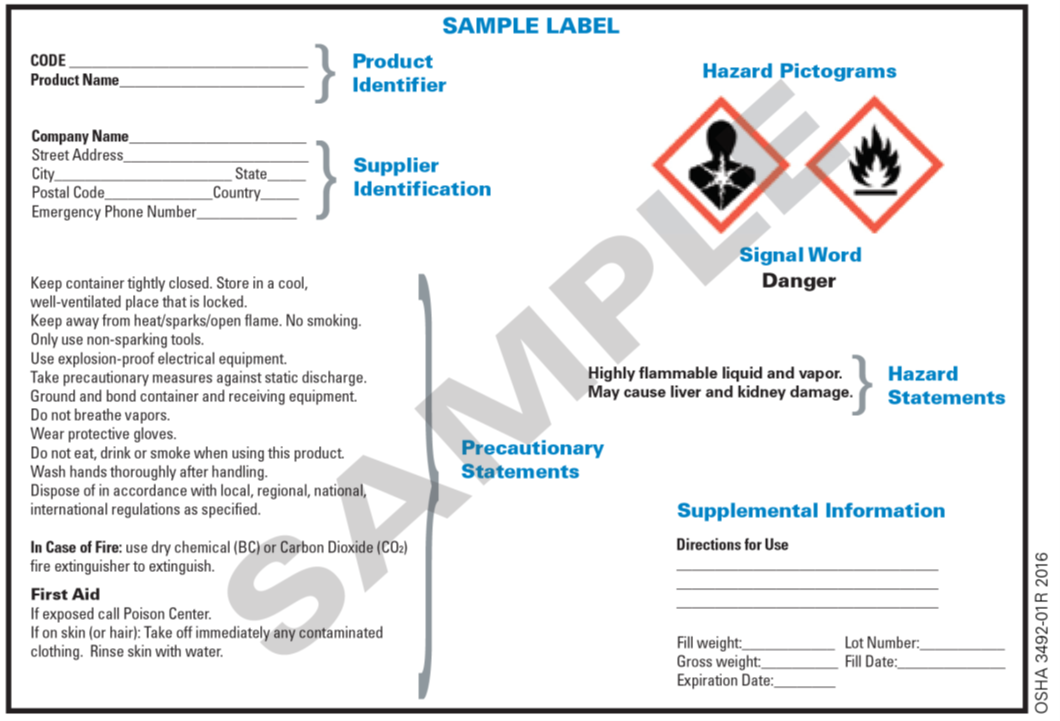
Requirements for Shipped Container and Workplace Labels Requirements for Shipped Container and Workplace Labels. As part of the 2012 revision of the Hazard Communication Standard, 29 CFR 1910.1200, OSHA adopted new hazardous chemical labeling requirements for shipped containers that align with the United Nations' Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
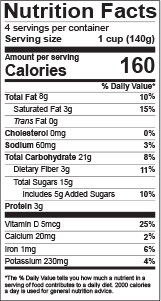
Fair Packaging and Labeling Act: Regulations Under Section 4 of the ... The Fair Packaging and Labeling Act (FPLA or Act), enacted in 1967, directs the Federal Trade Commission and the Food and Drug Administration to issue regulations requiring that all "consumer commodities" be labeled to disclose net contents, identity of commodity, and name and place of business of the product's manufacturer, packer, or distributor.
Pesticide Containers | US EPA Jan 03, 2022 · EPA’s test procedure for rinsing containers for the residue removal standard in 40 CFR 165.25(f). Container Handling Instructions on Pesticide Labels. The pesticide container regulations require pesticide labels to include statements identifying the container as nonrefillable or refillable and providing instructions on how to handle and clean it.
Proper Labeling Chemical Containers - Hazcom ... - OSHA Review OSHA requires every original chemical container to have a primary shipping label from the manufacturer with a few exceptions: Drugs for patient care, consumer chemicals, and pesticides, including disinfectants and dental unit waterline cleaners, are not subject to the labeling requirements.
Container Labeling: A Key to Compliance - Occupational Health & Safety Containers also must have the following identification on the container, label or tag [29 CFR 1910.1200 (f) (1)]: The identity of the hazardous chemical. Appropriate hazard warnings. The name and ...
Safety Considerations for Container Labels and Carton Labeling Design This guidance focuses on safety aspects of the application holder's container label and carton labeling design. It provides a set of principles and recommendations for ensuring that critical...
Labelling and packaging - ECHA - European Union CLP sets general requirements for labelling to ensure the safe use and supply of hazardous substances and mixtures. Certain labelling exemptions apply e.g. ...
Packaging and Labeling Regulations in the US and Canada CCCR 2001 is very prescriptive regarding its five main regulated classifications: toxicity, flammability, corrosivity, quick skin-bonding adhesives, and pressurized containers. If your product contains potentially hazardous chemicals, you should consult Reference Manual.
Compliance FAQs: Packaging and Labeling in the US | NIST title 19, united states code, chapter 4, section 1304 and 19 cfr 134, country of origin marking regulations require that every article of foreign origin (or its container) imported into the u.s. be marked in a conspicuous place as legibly, indelibly, and permanently as the nature of the article (or container) will permit, and in such a manner as …
1998 ap statistics Regulations require that product labels on containers of food that are available ... Specifically, if milk containers are labeled to have 128 fluid ounces.
Quality System Regulation Labeling Requirements | FDA Various sections of the QS regulation have an impact on labeling: Section 21 CFR 820.80 (b) requires the inspection and testing of incoming materials including labeling; and 21 CFR 820.70 (f ...





















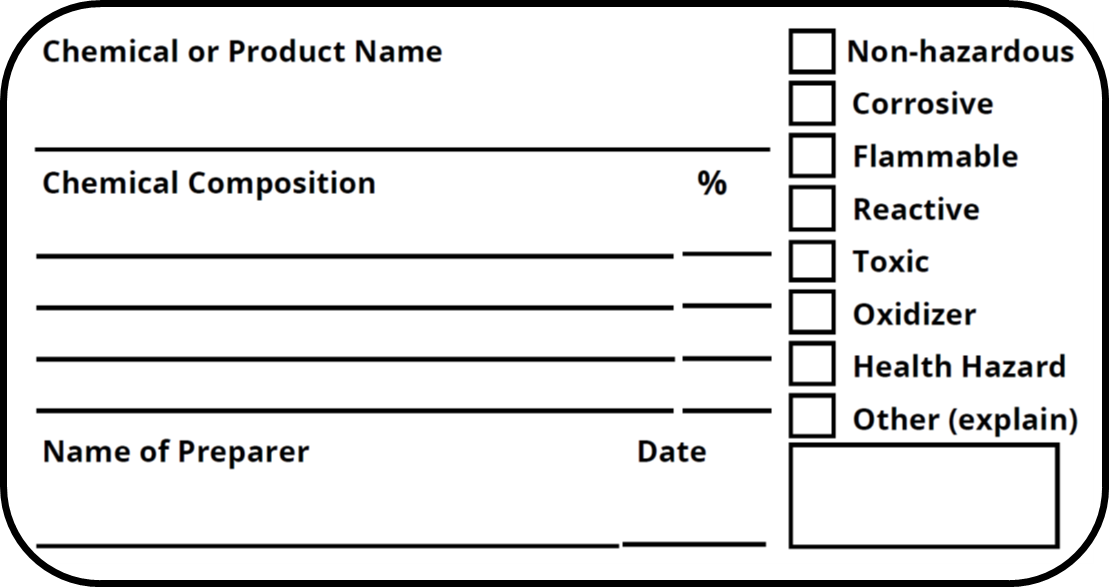







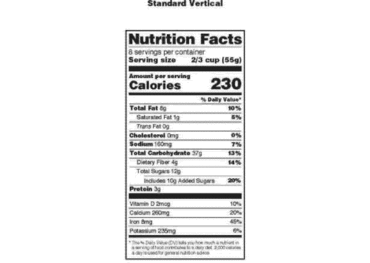
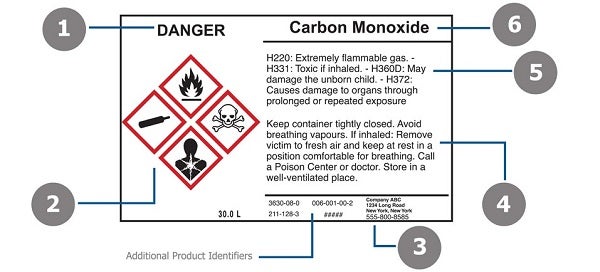


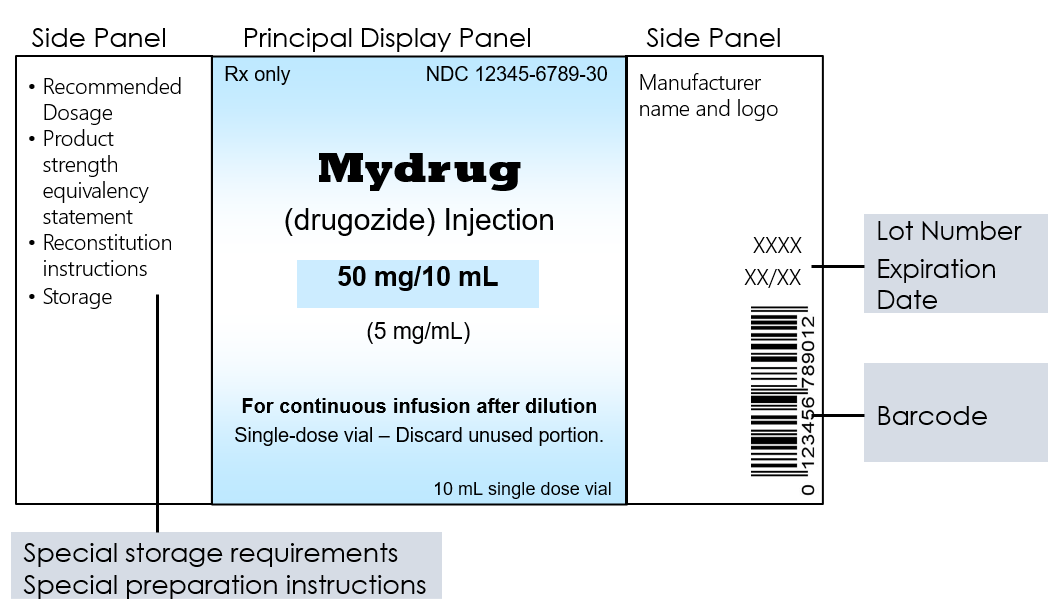

Post a Comment for "44 regulations require that product labels on containers"